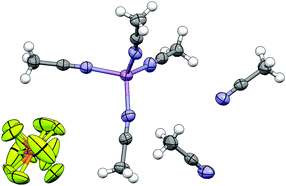
Solvation is a critical factor for determining the properties of electrolytes and lithium reagents, but only limited information is available about the coordination number for Li+ cations in solution with different solvents. The present manuscript examines the manner in which acetonitrile (AN) fully solvates Li+ cations. The results are also likely pertinent to other nitrile and dinitrile solvents. In particular, the crystal structure for a (AN)6:LiPF6 solvate is reported—this is the first 6/1 AN/Li solvate structure to be determined. The structure consists of Li+ cations fully solvated by four AN molecules (i.e., [(AN)4Li]+ species), uncoordinated PF6− anions and uncoordinated AN molecules (two per Li+ cation). This structure validates, in part, density functional theory (DFT) calculations which predict that there is little to no energetic benefit to coordinating Li+ cations with more than four AN solvent molecules.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...