Quinolinium and isoquinolinium ionic liquid crystals†
Abstract
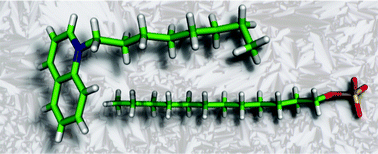
Ionic liquid crystals based on quinolinium and isoquinolinium salts were prepared by quaternization of quinoline and isoquinoline, respectively. In the first place the influence of the alkyl chain on the thermal behaviour was investigated. Two types of (iso)quinolinium salts were synthesized. Long-chained (iso)quinolinium cations (with chain lengths varying from C12H25 to C22H45), with the inclusion of the odd homologues C17H35 and C19H39, were combined with the spherical anions bromide, tetrafluoroborate (BF4−) and hexafluorophosphate (PF6−). Dodecyl sulfate, dioctyl-, dihexyl- and dicyclohexylsulfosuccinate anions allowed the introduction of anions with (long) alkyl chains. It is shown that a subtle balance between strong electrostatic forces and the degree of disorder originating from molten alkyl chains is crucial for mesophase formation. The influence of the anion size on the mesophase behaviour was studied. Smectic A phases were observed for the long chain analogues of the bromide, tetrafluoroborate and hexafluorophosphate salts. The minimum alkyl chain length needed to induce mesomorphism increases when the bromide is exchanged by more voluminous anions, while the transition temperatures are lowered. The introduction of an alkyl chain in the anionic part drastically changes the thermal behaviour. The dodecyl sulfate salts show smectic A phases for cations with much shorter chain lengths than the other anions. For the dioctylsulfosuccinate salts this trend is even more pronounced, as the N-methyl(iso)quinolinium dioctylsulfosuccinate salts exhibited enantiotropic smectic A phases at ambient temperature. Structural models for the packing of the quinolinium and isoquinolinium salts in the liquid-crystalline state are presented, as well as the crystal structures of N-octylquinolinium dodecyl sulfate and N-octylisoquinolinium bromide.

 Please wait while we load your content...
Please wait while we load your content...