9-O-N-aryl/arylalkyl amino carbonyl methyl substituted berberine analogues induce self-structure in polyadenylic acid†
Abstract
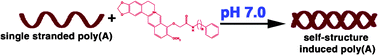
This manuscript describes the interaction of 9-O-substituted analogues of the plant alkaloid berberine with single stranded (ss) poly(A). Three new analogues of berberine with

 Please wait while we load your content...
Please wait while we load your content...