A green process for the epoxidation of dicyclopentadiene with aqueous H2O2 over highly efficient and stable HPW-NH2-SBA-15
Abstract
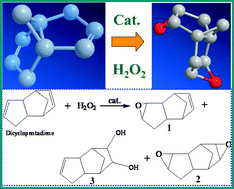
Dicyclopentadiene dioxide (2) was synthesized using an economic and green reaction by the direct oxidation of dicyclopentadiene (DCPD) with aqueous H2O2 over tungstic acid and aminopropyl-immobilized phosphotungstic acid on SBA-15, which was successfully obtained by the immobilization of the supported heteropolyacid (HPW) on the surface of the ordered mesoporous silica, SBA-15, by means of chemical bonding to aminosilane groups. The as-obtained materials were characterized by N2 sorption, transmission electron microscopy (TEM), X-ray diffraction (XRD), 31P-magic angle spinning (MAS) NMR and Raman spectroscopy. The 16% HPW-NH2-SBA-15 is highly efficient in the reaction with a DCPD conversion of 100% and (2) selectivity up to 97%. It is interesting that this material could be reused six times without any significant loss of activity and selectivity. The good stability can be attributed to the strong interaction between the amino groups on the surface of SBA-15 and HPW anions.

 Please wait while we load your content...
Please wait while we load your content...