Synthesis, characterization and DNA binding properties of rutin–iron complex
Abstract
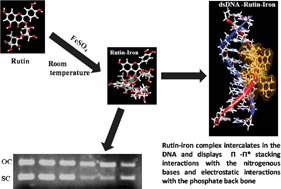
A rutin–iron complex was prepared at room temperature and characterized using elemental analysis, IR spectroscopy and UV-visible spectroscopy to elucidate its structure. The DNA binding properties of rutin and the rutin–iron complex have been investigated using electronic absorption spectroscopy and agarose gel electrophoresis. The gradual reduction in the absorption peak observed for both rutin and the rutin–iron complex with increasing concentration of DNA indicates an intercalative mode of interaction. The binding constant (Kb) for the rutin–iron complex was three fold greater than that for rutin. Rutin exhibits a tendency to degrade the plasmid DNA unlike the rutin–iron complex because of its ability to introduce torsional stress in the DNA double helix. The additional electrostatic interaction between the cationic rutin–iron complex and the anionic DNA does not contribute to any torsional strain and hence no DNA shearing was observed. Molecular docking studies also confirmed the intercalative mode of interaction for both the compounds. These results indicate the potential of the rutin–iron complex as an anti-cancer agent.

 Please wait while we load your content...
Please wait while we load your content...