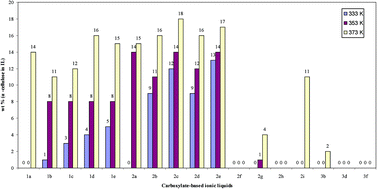
The dissolution of cellulose allows easier processing of this important biogenic feedstock. For this, ionic liquids have been proposed. Carboxylate-based ionic liquids were identified as the most promising lead towards a high dissolution property. Three homologous series of all 27 combination of the three cations: 1-ethyl-3-methylimidazolium, 1,3-dimethylimidazolium, and N,N-diethyl-N,N-dimethylammonium with nine carboxylates as anions were synthesised. The cellulose solubilities of the 17 ionic liquid compounds (liquid below 373 K) were measured. Up to 18 wt% for 1-ethyl-3-methyl-imidazolium propionate was achieved, slightly higher than when using acetate as the anion. Generally, the solubilities determined for carboxylate-based ionic liquids with imidazolium cations were found to be in the same range, whereas those with quaternary ammonium cations were found to be poor solvents for cellulose. Dicarboxylates gave higher solubilities compared to monocarboxylates. Regenerated ionic liquids had no apparent difference to fresh ones.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...