Iodine catalyzed four-component reaction: a straightforward one-pot synthesis of functionalized pyrroles under metal-free conditions†
Abstract
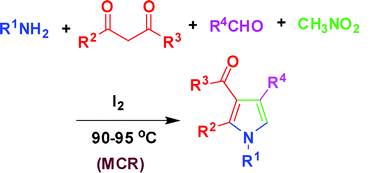
An iodine-catalyzed four-component reaction of 1,3-dicarbonyl compounds,
- This article is part of the themed collection: Organic Collection: from theory to synthesis, from molecules to materials, catalysis and beyond

 Please wait while we load your content...
Please wait while we load your content...