Catalytic versus stoichiometric dehydrocoupling using main group metals
Abstract
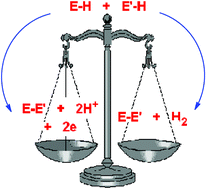
A primary factor influencing catalyticversus stoichiometric behaviour of molecular main group species in homogeneous dehydrocoupling reactions is the redox stability of the metal centre. Thus, only in the case of redox-stable metals has catalytic behaviour so far been observed, through genuinely hydrogenic coupling (E–H + E′–H → E–E′ + H2), whereas for redox-unstable metals oxidative dehydrocoupling is seen (E–H + E′–H → E–E′ + 2H+ + 2e). The mechanisms of catalytic P–H/P–H and B–H/N–H dehydrocoupling involving main group systems are closely related to d0 transition metal counterparts and produce a similar range of products, although the main group systems reported so far are not as active as the most active transition metal
- This article is part of the themed collection: Organic Collection: from theory to synthesis, from molecules to materials, catalysis and beyond

 Please wait while we load your content...
Please wait while we load your content...