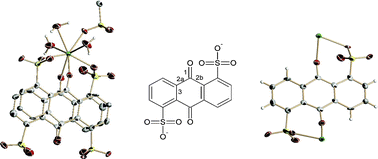
With the correct choice of the solvothermal conditions, we have achieved the unprecedented in situ formation of the free radical form of the anthraquinone-1,5-disulfonate molecule, and its favorable organization. The semiquinone radicals are coordinated to rare-earth cations to produce a 2D framework with a very high charge mobility and electric conductivity through the π–π-interactions. The existence of AQDS3−˙ anion radicals is proven on the base of: i) the electrical neutrality: elemental analyses for the different lanthanide RPF-8 bulks, the maximum residual electron densities in the structure, rule out the existence of any other neutralizing ion, ii) the geometrical modifications in the antraquinone molecules, and iii) although less definitive, due to the low magnetic moment μ = 0.39 μB, the exhibited paramagnetism for the La (3+) with no unpaired electrons.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...