Conjugate hydrotrifluoromethylation of α,β-unsaturated acyl-oxazolidinones: synthesis of chiral fluorinated amino acids†
Abstract
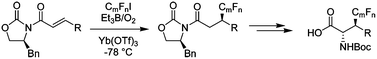
A novel conjugate hydrofluoroalkylation of α,β-unsaturated acyl-oxazolidinones is described. Using this method, enantiomerically pure β-trifluoromethylated

 Please wait while we load your content...
Please wait while we load your content...