Total synthesis of indole-3-acetonitrile-4-methoxy-2-C-β-d-glucopyranoside. Proposal for structural revision of the natural product†
Abstract
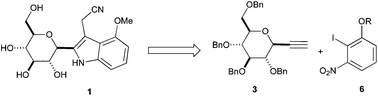
Indole-3-acetonitrile-4-methoxy-2-C-β-D-glucopyranoside (1), a novel C-glycoside from Isatis indigotica with important cytotoxic activity, has been prepared in ten steps from ethynyl-β-C-glycoside 3 and

 Please wait while we load your content...
Please wait while we load your content...