Highly regio-, diastereo- and enantioselective one-pot gold/chiral Brønsted acid-catalysed cascade synthesis of bioactive diversely substituted tetrahydroquinolines†
Abstract
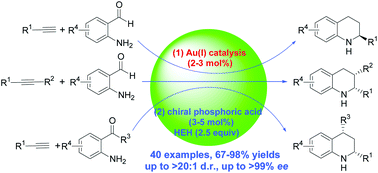
One-pot sequential asymmetric reactions of aminobenzaldehydes or aminophenones with

 Please wait while we load your content...
Please wait while we load your content...