Kinetic and computational evidence for an intermediate in the hydrolysis of sulfonate esters†
Abstract
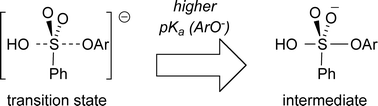
The hydrolytic reactions of sulfonate esters have previously been considered to occur by concerted mechanisms. We now report the observation of a break in a Brønsted correlation for the alkaline hydrolysis of aryl benzenesulfonates. On either side of a break-point, βleaving group values of −0.27 (pKa < 8.5) and −0.97 (pKa > 8.5) are measured. These data are consistent with a two-step mechanism involving a pentavalent intermediate that is also supported by QM/MM calculations. The emerging scenario can be explained by the combined effect of a strong nucleophile with a poor leaving group that compel a usually concerted reaction to favour a stepwise process.

 Please wait while we load your content...
Please wait while we load your content...