Direct catalytic asymmetric synthesis of highly functionalized (2-ethynylphenyl)alcohols via Barbas–List aldol reaction: scope and synthetic applications†‡
Abstract
A high-yielding asymmetric synthesis of functionalized (2-ethynylphenyl)alcohols 5/6 with good diastereo- and enantioselectivities was achieved by Barbas–List aldol (BLA) reaction of 2-alkynylbenzaldehydes 1 with various
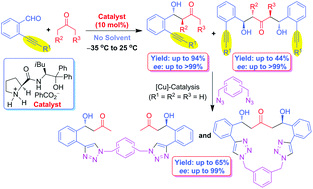
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...