A strategy for the synthesis of the fargenone/fargenin family of natural products: synthesis of the tricyclic core†
Abstract
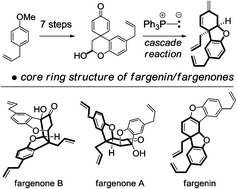
A synthesis of the core ring structure of the fargenin/fargenone family of natural products is presented. The general strategy is based upon biosynthetic speculation and exploits a cascade reaction, which transforms a spirocyclic dienone into the core ring system via a deprotonation–oxy-Michael–Wittig olefination sequence. This study represents the first synthesis work towards this family of natural products.

 Please wait while we load your content...
Please wait while we load your content...