Enantioselective synthesis of gabapentin analogues via organocatalytic asymmetric Michael addition of α-branched aldehydes to β-nitroacrylates†‡
Abstract
Michael addition reaction of α-branched
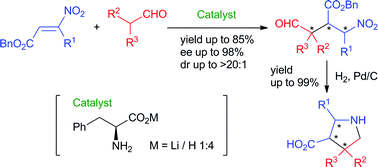
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...