Enantioselective direct aldol reaction of α-keto esters catalyzed by (Sa)-binam-d-prolinamide under quasi solvent-free conditions†‡
Abstract
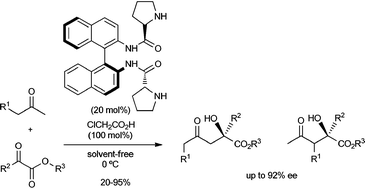
(Sa)-Binam-D-prolinamide (20 mol%), instead of (Sa)-binam-L-prolinamide, in combination with chloroacetic acid (100 mol%) is an efficient organocatalyst for the direct aldol reaction between α-keto esters as electrophiles and alkyl and α-functionalised ketones, under quasi solvent-free conditions, providing access to highly functionalised chiral quaternary γ-keto α-hydroxyesters with up to 92% ee.

 Please wait while we load your content...
Please wait while we load your content...