The synthesis of the tricyclic angular chromone structure originally assigned to aspergillitine is reported. The synthesis was achieved in 11 steps and 15% overall yield from 2,4-dihydroxypropiophenone, through the intermediacy of 2,3-dimethyl-7-hydroxychromen-4-one. Construction of the nitrogen-bearing heterocyclic ring entailed a Stille cross-coupling reaction with n-Bu3SnCH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, followed by double bond isomerization, oximation of the chromone carbonyl, and a final microwave-assisted electrocyclization of the thus formed 6π-electron aza-triene system.
CH2, followed by double bond isomerization, oximation of the chromone carbonyl, and a final microwave-assisted electrocyclization of the thus formed 6π-electron aza-triene system.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, followed by double bond isomerization, oximation of the
CH2, followed by double bond isomerization, oximation of the 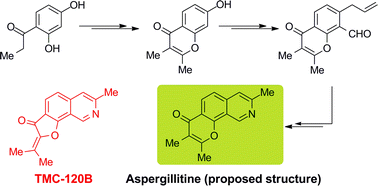

 Please wait while we load your content...
Please wait while we load your content...