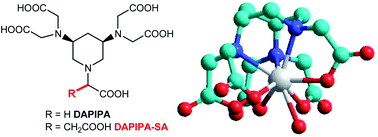
Lorenzo Tei, Gabriele A. Rolla, Giuseppe Gugliotta and Mauro Botta
Org. Biomol. Chem., 2012,10, 2525-2527
DOI:
10.1039/C2OB07154A,
Communication
The core structure of