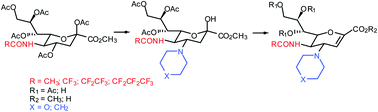
A simple protocol for the synthesis of N-perfluoroacylated and N-acylated glycals of neuraminic acid, with a secondary cyclic amine (morpholine or piperidine) at the 4α position, has been set-up, starting from peracetylated N-acetylneuraminic acid methyl ester that undergoes, sequentially to its direct N-transacylation followed by a C-4 amination, a β-elimination, and a selective hydrolysis of the ester functions, without affecting the sensitive perfluorinated amide.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Please wait while we load your content...
Please wait while we load your content...