Synthesis of N-substituted ε-hexonolactams as pharmacological chaperones for the treatment of N370S mutant Gaucher disease†
Abstract
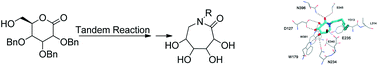
A series of N-substituted ε-hexonolactams have been designed and prepared by a concise route with a tandem ring-expansion reaction as the key step. Some of the N-substituted ε-hexonolactams show better enhancements to N370S mutant β-glucocerebrosidase activity than NB-DNJ and NN-DNJ. Both the experimental results and computational studies highlight the importance of the

 Please wait while we load your content...
Please wait while we load your content...