Co-delivery of doxorubicin and siRNA using octreotide-conjugated gold nanorods for targeted neuroendocrine cancer therapy†
Abstract
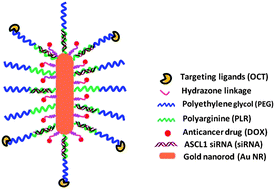
A multifunctional gold (Au) nanorod (NR)-based nanocarrier capable of co-delivering small interfering RNA (siRNA) against achaete-scute complex-like 1 (ASCL1) and an anticancer drug (doxorubicin (DOX)) specifically to neuroendocrine (NE) cancer cells was developed and characterized for combined chemotherapy and siRNA-mediated gene silencing. The Au NR was conjugated with (1) DOX, an anticancer drug, via a pH-labile hydrazone linkage to enable pH-controlled drug release, (2) polyarginine, a cationic polymer for complexing siRNA, and (3) octreotide (OCT), a tumor-targeting ligand, to specifically target NE cancer cells with overexpressed somatostatin receptors. The Au NR-based nanocarriers exhibited a uniform size distribution as well as pH-sensitive drug release. The OCT-conjugated Au NR-based nanocarriers (Au–DOX–OCT, targeted) exhibited a much higher cellular uptake in a human carcinoid cell line (BON cells) than non-targeted Au NR-based nanocarriers (Au–DOX) as measured by both flow cytometry and confocal laser scanning microscopy (CLSM). Moreover, Au–DOX–OCT–ASCL1 siRNA (Au–DOX–OCT complexed with ASCL1 siRNA) resulted in significantly higher gene silencing in NE cancer cells than Au–DOX–ASCL1 siRNA (non-targeted Au–DOX complexed with ASCL1 siRNA) as measured by an immunoblot analysis. Additionally, Au–DOX–OCT–ASCL1 siRNA was the most efficient nanocarrier at altering the NE phenotype of NE cancer cells and showed the strongest anti-proliferative effect. Thus, combined chemotherapy and RNA silencing using NE tumor-targeting Au NR-based nanocarriers could potentially enhance the therapeutic outcomes in treating NE cancers.

 Please wait while we load your content...
Please wait while we load your content...