Mechanical properties of graphene oxides
Abstract
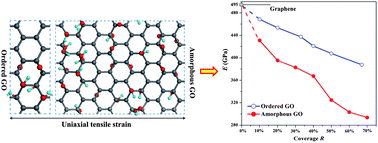
The mechanical properties, including the Young's modulus and intrinsic strength, of graphene oxides are investigated by first-principles computations. Structural models of both ordered and amorphous graphene oxides are considered and compared. For the ordered graphene oxides, the Young's modulus is found to vary from 380 to 470 GPa as the coverage of oxygen groups changes, respectively. The corresponding variations in the Young's modulus of the amorphous graphene oxides with comparable coverage are smaller at 290–430 GPa. Similarly, the ordered graphene oxides also possess higher intrinsic strength compared with the amorphous ones. As coverage increases, both the Young's modulus and intrinsic strength decrease monotonically due to the breaking of the sp2 carbon network and lowering of the energetic stability for the ordered and amorphous graphene oxides. In addition, the band gap of the graphene oxide becomes narrower under uniaxial tensile strain, providing an efficient way to tune the electronic properties of graphene oxide-based materials.

 Please wait while we load your content...
Please wait while we load your content...