Theoretical analysis of the effect of particle size and support on the kinetics of oxygen reduction reaction on platinum nanoparticles†
Abstract
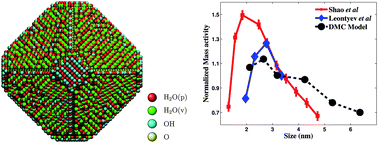
We perform a first-principles based computational analysis of the effect of particle size and support material on the electrocatalytic activity of platinum nanoparticles. Using a mechanism for oxygen reduction that accounts for electric field effects and stabilization from the water layer on the (111) and (100) facets, we show that the model used agrees well with linear sweep voltammetry and rotating ring disk electrode experiments. We find that the per-site activity of the nanoparticle saturates for particles larger than 5 nm and we show that the optimal particle size is in the range of 2.5–3.5 nm, which agrees well with recent experimental work. We examine the effect of support material and show that the perimeter sites on the metal–support interface are important in determining the overall activity of the nanoparticles. We also develop simple geometric estimates for the activity which can be used for determining the activity of other particle shapes and sizes.
 Please wait while we load your content...
Please wait while we load your content...