Covering: up to 2011
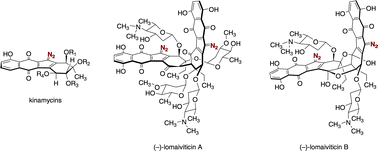
This review presents a comprehensive survey of all aspects of the kinamycins and lomaiviticins, potent antiproliferative antimicrobial metabolites isolated from various strains of Streptomyces and Salinispora. The kinamycins and lomaiviticins contain a diazotetrahydrobenzo[b]fluorene (diazofluorene) functional group, which is unique among known natural products. This review begins with an account of the studies leading to the final (correct) structure determination of the kinamycins, which were originally proposed to contain an N-cyano carbazole function. This is followed by a discussion of biosynthetic studies, which established the polyketide nature of the kinamycins. Descriptions of four completed syntheses of various kinamycins, synthetic studies toward the lomaiviticins, syntheses of the carbohydrates of the lomaiviticins, and syntheses of structurally-related metabolites, are then presented. A survey of chemical biological investigations, including in vitro reactivity studies, which indicate that the kinamycins and lomaiviticins may form reactive ortho-quinone methide or free radical intermediates in vivo, is presented. Finally, a selection of structurally-related metabolites are described.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...