Syntheses and structures of new dicopper(ii) complexes bridged by N-(2-hydroxyphenyl)-N′-(3-aminopropyl)oxamide: DNA-binding properties and cytotoxic activities†
Abstract
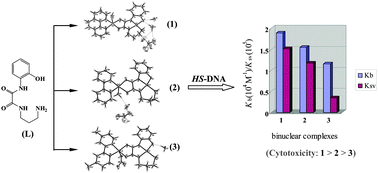
Three new μ-oxamido-bridged dicopper(II) complexes with the formulae [Cu2(papo)(phen)(H2O)](ClO4)·2H2O (1), [Cu2(papo)(bpy)(H2O)](NO3)·H2O (2) and [Cu2(papo)(Me2bpy)(NO3)]·H2O (3) [H3papo = N-(2-hydroxyphenyl)-N′-(3-aminopropyl)oxamide; phen = 1,10-phenanthroline; bpy = 2,2′-bipyridine; Me2bpy = 4,4′-dimethyl-2,2′-bipyridine], have been synthesized and characterized by elemental analyses, molar conductance measurements, IR and electronic spectra studies and X-ray single-crystal diffraction. In the three dicopper(II) complexes, the coordination environments around the copper(II) atoms are different: Cu1 and Cu2 atoms have a square-planar and a square- pyramidal geometry, respectively. The Cu⋯Cu distances through the cis-papo3−-bridge in complexes 1, 2 and 3 are 5.1936(7), 5.1967(7) and 5.1919(15) Å, respectively. In the crystals of the three dicopper(II) complexes, there are abundant hydrogen bonds contributing to the complicated supramolecular structure. The reactivity towards DNA, and cytotoxic studies show that all three dicopper(II) complexes can bind to herring sperm DNA (HS-DNA) in the mode of intercalation, and the cytotoxic activities are consistent with their DNA-binding abilities, following the order of 1 > 2 > 3. The influence of structural variation of the terminal ligands in the dinuclear complexes on the DNA-binding property and cytotoxic activity is preliminarily discussed.

 Please wait while we load your content...
Please wait while we load your content...