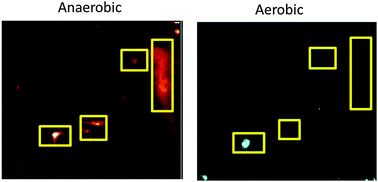
Metals are critical and dynamic components of biochemistry. To understand their roles, we greatly need tools to identify the ligands that bind them within the complexity of natural systems. This work describes the development of methods that not only detect and distinguish metals, but also characterize the proteins that bind them. We describe an approach to expose, identify and quantify metalloproteins in complex mixtures by sequential non-denaturing 2D-gel electrophoresis (2D GE)/X-ray Fluorescence (XRF) and tandem mass spectrometry (MS/MS) in the same spot of a sample. We first apply the development of 2D GE/XRF to Shewanella oneidensis MR-1, a well-studied system, and verify our electrophoretic approach. Then, we identified a novel periplasmic zinc protein in Pseudomonas aeruginosa PAO1 through 2D GE/XRF followed by MS/MS. The identity and function of this protein was verified through a gene mutation experiment.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...