Synthesis and in vitro cytotoxicity of cis,cis,trans-diamminedichloridodisuccinatoplatinum(iv)–peptidebioconjugates
Abstract
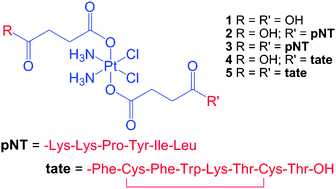
The synthesis and characterization of four Pt(IV)–
* Corresponding authors
a
Dipartimento di Scienze dell'Ambiente e della Vita, Università del Piemonte Orientale “Amedeo Avogadro”, Viale T. Michel 11, I-15121 Alessandria, Italy
E-mail:
mauro.ravera@mfn.unipmn.it
Fax: +39 0131 360250
Tel: +39 0131 360252
b Lehrstuhl für Anorganische Chemie I – Bioanorganische Chemie, Fakultät für Chemie und Biochemie, Ruhr-Universität Bochum, Universitätsstrasse 150, D-44801 Bochum, Germany
The synthesis and characterization of four Pt(IV)–

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
