Improvement of the trypanocidal activity of 3-arylthiophene farnesyltransferase inhibitors by modulation of their 3-aryl group†
Abstract
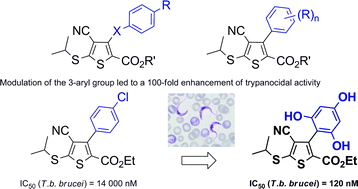
Tetrasubstituted 3-arylthiophenes represent a new class of potential
* Corresponding authors
a
CNRS, Institut de Chimie des Substances Naturelles, Centre de Recherche de Gif, Avenue de la Terrasse, 91198 Gif-sur-Yvette Cedex, France
E-mail:
joelle.dubois@icsn.cnrs-gif.fr
Fax: +33 1 69 07 72 47
Tel: +33 1 69 82 30 58
b Muséum National d'Histoire Naturelle, UMR 7245 CNRS, Département RDDM, CP52, 57 rue Cuvier, 75005 Paris, France
Tetrasubstituted 3-arylthiophenes represent a new class of potential

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
D. Bosc, S. Lethu, E. Mouray, P. Grellier and J. Dubois, Med. Chem. Commun., 2012, 3, 1512 DOI: 10.1039/C2MD20197F
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
