Abstract
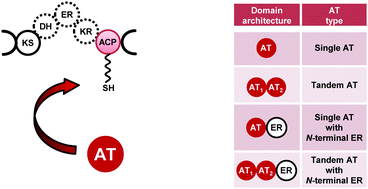
Polyketides (PKs) are a class of natural products with highly diverse structures and bioactivities. Important clinical antibiotics like tetracycline, erythromycin, mupirocin, and dalfopristin (a component of Synercid®) belong to this compound class. The diversity of PK structures depends on their assembly process, for which multifunctional enzymes, the modular polyketide synthases (PKSs), are required. These megasynthases are organized in modules, consisting of integrated enzymatic domains, which catalyze the individual reaction steps in PK biosynthesis. Here, we focus on a subset of PKSs, the trans-AT type modular PKSs and their discrete acyltransferases (ATs). In contrast to the classical cis-AT PKSs which have AT domains within the megasynthases, the trans-AT PKSs recruit discrete ATs, which are encoded by separate genes. ATs are required for the selection of precursors and their loading to acyl carrier protein (ACP) domains of the PKS. Although, the number of identified trans-AT PKS gene clusters increased over the last decade, the functional analysis of the discrete ATs in those pathways was carried out only for a few examples. In these cases – excluding ATs accepting ACP-linked extenders – transfer of malonyl-CoA was observed. This suggested a strict malonyl-CoA specificity of the in trans acting ATs. Only recently, a discrete AT, which selects freestanding ethylmalonyl-CoA and uses this substrate for the direct loading of a specific ACP domain of a kirromycin PKS, was described. Furthermore, this represents a novel mechanism of incorporation of branched structures into polyketides. The identification of the AT motifs responsible for the substrate recognition and ACP docking are of particular importance for understanding and modification of such systems. Here, we provide a summary of information gained from experimental studies on discrete ATs involved in polyketide biosynthesis until the end of 2011.

 Please wait while we load your content...
Please wait while we load your content...