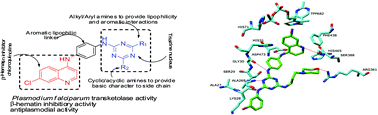
Analogues of a novel class of hybrid 4-anilinoquinoline triazines have been synthesized with the aim of identifying the compounds with improved antimalarial activity preserving the potency of parent drug chloroquine (CQ). All the synthesized molecules were evaluated in vitro for their antimalarial activity against chloroquine-sensitive 3D7 and chloroquine-resistant K1 strains of P. falciparum. Molecules were also screened for their cytotoxicity towards VERO cell line. Sixteen compounds (17, 19, 26, 27, 29, 31, 32, 33, 35, 36, 37, 39, 40, 49, 50, and 52) exhibited excellent antimalarial activity with IC50 values ranging from 1.36–4.63 ng ml−1 and were also found to be nontoxic with good selectivity index. In silico activity prediction as well as enzyme inhibitory activity against P. falciparumtransketolase reveals that the molecules are also good inhibitors of the enzymeP. falciparumtransketolase. The compound 52 showed good in vivo activity by oral route and resulted in survival of 3 out of 5 mice till day 28.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...