Proteolysis of beta-galactosidase following SigmaB activation in Bacillus subtilis†
Abstract
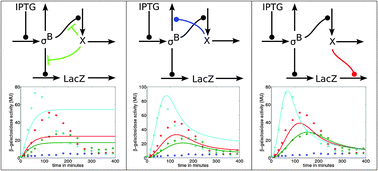
In Bacillus subtilis the σB mediated general stress response provides protection against various environmental and energy related stress conditions. To better understand the general stress response, we need to explore the mechanism by which the components interact. Here, we performed experiments in B. subtilis wild type and mutant strains to test and validate a mathematical model of the dynamics of σB activity. In the mutant strain BSA115, σB transcription is inducible by the addition of IPTG and negative control of σB activity by the anti-sigma factor RsbW is absent. In contrast to our expectations of a continuous β-galactosidase activity from a ctc::lacZ fusion, we observed a transient activity in the mutant. To explain this experimental finding, we constructed mathematical models reflecting different hypotheses regarding the regulation of σB and β-galactosidase dynamics. Only the model assuming instability of either ctc::lacZ mRNA or β-galactosidase

 Please wait while we load your content...
Please wait while we load your content...