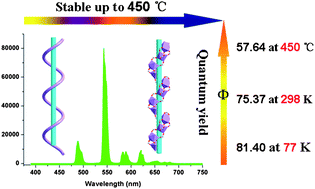
New luminescent lanthanide-carboxylate frameworks, formulated as [Ln4(m-BDC)6(H2O)4(DMF)]·(H2O)2·(DMF) (Ln = Eu 1; Gd 2; Tb 3) (m-H2BDC = 1,3-benzenedicarboxylic acid) have been solvo(hydro)thermally synthesized. Single-crystal X-ray diffraction studies reveal that compounds 1–3 are isomorphous and each displays a layered structure constructed by helical lanthanide-carboxylate chains. DFT and TD-DFT calculations were conducted in detail to discuss the energy levels, including the HOMO, LUMO, singlet and triplet energies of the m-H2BDC ligand. The results indicate that the m-BDC2− dianion acts as an antenna chromophore that is able to efficiently absorb and transfer energy to the lanthanide ions. Compounds 1–3 all exhibit high thermostability and are stable up to 450 °C, as confirmed by the TGA and temperature-dependent PXRD experiments. Compounds 1 and 3 are strong luminescent materials. Compound 3 is among some of the best examples of luminescent lanthanide compounds, with highly efficient emission quantum yields of 81.40% at 77 K, 75.37% at room temperature and 56.64% after calcination at 450 °C, respectively. In addition, the energy transfer processes for compounds 1 and 3 have also been studied and illustrated in detail.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...