Substitution effect of manganese for iron in “114” YBaFe4O7 ferrite: structure, magnetism and oxygen hyperstoichiometry
Abstract
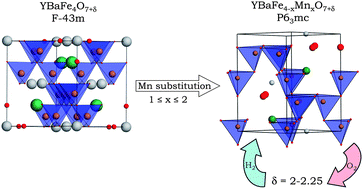
The study of the ferrites YBaFe4−xMnxO7 shows that the substitution of Mn2+ for Fe2+ in YBaFe4O7 stabilizes the hexagonal symmetry at the expense of the cubic one for 1 ≤ x ≤ 2. Using combined measurements of a.c. and d.c. magnetization, we establish that Mn substitution for Fe in YBaFe4O7 leading to a cationic disorder induces a spin glass behaviour. An important characteristic of these Mn substituted ferrites deals with their ability to absorb and desorb reversibly oxygen, leading to the

 Please wait while we load your content...
Please wait while we load your content...