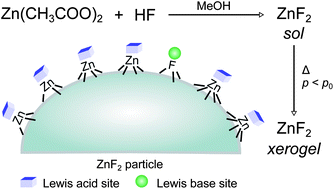
The fluorolytic sol–gel route sets a milestone in the development of synthesis methods for nanoscopic fluoride materials. They exhibit fundamentally distinct properties in comparison to classically prepared metal fluorides. To broaden this area, we report in this paper the first fluorolytic sol–gel synthesis of ZnF2. The obtained sol was studied with dynamic light scattering (DLS). The dried ZnF2 xerogel was investigated with elemental analysis, thermal analysis, powder X-ray diffraction (XRD), solid-state MAS NMR, and N2 adsorption–desorption measurements. The characterisations revealed a remarkably high surface area of the sol–gel prepared ZnF2. To determine key parameters deciding its prospects in future catalytic applications, we studied the surface acidity–basicity by using in situ FTIR with different probe molecules. Compared to the previously established MgF2, weaker Lewis acid sites are predominant on the surface of ZnF2 with some base sites, indicating its potential as a heterogeneous catalyst component. In short, we believe that the successful synthesis and detailed characterisation of nanoscopic ZnF2 allow follow-up work exploring its applications, and will lead to studies of more metal fluorides with similar methods.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...