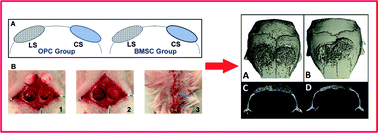
Scaffold architecture has exhibited significant impact on bone tissue regeneration; however, few studies have tried to address the relationship between scaffold architecture and structural organization of newly generated bones. The aim of this study was to investigate the effect of scaffold architecture on the structural organization of a newly formed bone using a two-hole mouse calvarial defect model. Evaluation of bone formation indicated although the new bone was formed in both types of scaffolds, their structure was closely related to the microstructure of scaffolds. Both micro-CT and histology data have shown that, morphologically, cortical-like bone was formed in the lamellar scaffold (LS) group while trabecular-like bone was found in the cellular scaffold (CS) group. This finding expands the understanding of the role of scaffold architecture during bone regeneration. Moreover, early stage bone formation was observed in defects filled with LS regardless of donor cell types at day 14 while no bone formation was found in the CS group. In addition, when calvarial osteoprogenitor cells (OPCs) and bone marrow stromal cells (BMSCs) were used as the donor cells respectively, it was found that BMSCs not only induced better host integration by recruiting more host cell infiltration, but also better supported bone marrow and vascularization reconstruction in the defects. These results collectively suggest that the scaffold structure design strongly dictates the structural organization of the new bone which may eventually influence the bone healing outcome.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...