Synthesis of graphene-multiwalled carbon nanotubes hybrid nanostructure by strengthened electrostatic interaction and its lithium ion battery application
Abstract
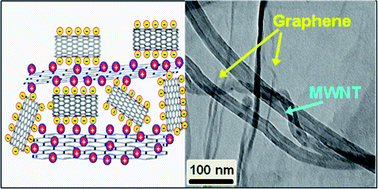
We report a novel way of synthesizing
* Corresponding authors
a
Alternative Energy and Nanotechnology Laboratory (AENL), Nanofunctional Materials Technology Center (NFMTC), Department of Physics, Indian Institute of Technology Madras, Chennai-600036, India
E-mail:
ramp@iitm.ac.in
Fax: +91-44-22570509
Tel: x91-44-22574862
b Centre for Fuel Cell Technology (ARCI), IIT Madras Research Park, Phase 1, 2nd Floor 6, Kanagam Road Taramani, Chennai 600 113, India
We report a novel way of synthesizing

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
B. P. Vinayan, R. Nagar, V. Raman, N. Rajalakshmi, K. S. Dhathathreyan and S. Ramaprabhu, J. Mater. Chem., 2012, 22, 9949 DOI: 10.1039/C2JM16294F
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
