Multi-addressable molecular switches based on photochromic diarylethenes bearing a rhodamine unit
Abstract
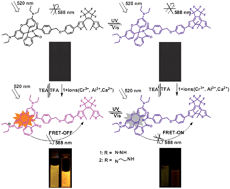
Two novel diarylethene derivatives bearing a rhodamine unit have been successfully synthesized. Their unique multi-addressable switching characteristics as induced by chemical and optical dual inputs stimulation were observed using UV and FL measurements. The two diarylethenes showed excellent photochormism with alternating UV/vis light irradiation. Addition of

 Please wait while we load your content...
Please wait while we load your content...