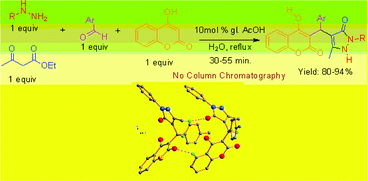
A combinatorial library of benzylpyrazolyl coumarin derivatives have been synthesized by a green one-pot four-component reaction between aryl hydrazine/hydrazine hydrate (1), ethyl acetoacetate (2), aromatic aldehydes (3) and 4-hydroxycoumarin (4). Molecular scaffolds which assimilate bio-active 3-benzylsubstituted 4-hydroxycoumarins as well as a pyrazolone ring in a single nucleus may be worthwhile molecules from a biological point of view. The reactions were performed in water and employed glacial acetic acid as the right choice of catalyst, and was demonstrated to be the key for rendering the reaction possible and obtained good to excellent yields under reflux conditions within a short period of time. The crystal structures of (R/S)-benzylpyrazolyl coumarin, easily produced by a chromatography-free highly product-selective reaction, were explored by means of single crystal X-ray diffraction analysis and the main intra- and intermolecular interactions perceivable through crystal structure analysis. The presence of a strong intramolecular hydrogen bond was confirmed. In addition, the whole crystal structure consists of other intermolecular hydrogen bonds, such as CH⋯O and aromatic CH⋯π interactions between R- and S-molecules and CH⋯O, NH⋯O and aromatic CH⋯π interactions among only R-molecules and S-molecules in the asymmetric unit.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...