Magnetic separation of fatty acids with iron oxide nanoparticles and application to extractive deacidification of vegetable oils†
Abstract
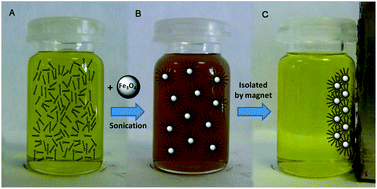
The magnetically assisted removal of fatty acids from organic solutions and vegetable oils using iron oxide magnetic nanoparticles (MNPs) was investigated. The effect of contact time and concentration on the adsorption of oleic acid from ethanol–hexane solutions was investigated at room temperature using equilibrium batch experiments. The results showed that the adsorption is rapid (<2 h) and follows a pseudo-second-order model. The adsorption isotherm was found to follow the Langmuir model and the maximum adsorption capacity of oleic acid was determined to be 125 mg g−1. FTIR analyses of the magnetically separated MNPs demonstrated the covalent binding of the carboxylic group to the particle surface in a bidentate/bridging manner. Thermogravimetric analyses showed that the adsorption capacity of MNPs is very similar for the most common fatty acids in vegetable oils (palmitic, stearic, oleic and linoleic acids). Desorption of fatty acids was readily achieved upon basic treatment and the regenerated magnetite nanoparticles were found to be recyclable for repeated use. The separation of fatty acids from olive and sunflower oils was investigated without added solvent. MNPs were found to remove up to 85% of the fatty acids in the oil within 2 h with a 10 wt% load at room temperature, without alteration of the pigment composition.

 Please wait while we load your content...
Please wait while we load your content...