Overall photocatalytic water splitting with NiOx–SrTiO3 – a revised mechanism
Abstract
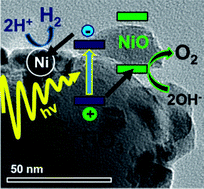
NiOx (0 < x < 1) modified SrTiO3 (STO) is one of the best studied photocatalysts for overall water splitting under UV light. The established mechanism for this and many other NiOx containing catalysts assumes water oxidation to occur at the early transition metal oxide and water reduction at NiOx. Here we show that NiOx–STO is more likely a three component Ni–STO–NiO catalyst, in which STO absorbs the light, Ni reduces protons, and NiO oxidizes water. This interpretation is based on systematic H2/O2 evolution tests of appropriately varied catalyst compositions using oxidized, chemically and photochemically added nickel and NiO nanoparticle cocatalysts. Surface photovoltage (SPV) measurements reveal that Ni(0) serves as an electron trap (site for water reduction) and that NiO serves as a hole trap (site for water oxidation). Electrochemical measurements show that the overpotential for water oxidation correlates with the NiO content, whereas the water reduction overpotential depends on the Ni content. Photodeposition experiments with NiCl2 and H2PtCl6 on NiO–STO show that electrons are available on the STO surface, not on the NiO particles. Based on photoelectrochemistry, both NiO and Ni particles suppress the Fermi level in STO, but the effect of this shift on catalytic activity is not clear. Overall, the results suggest a revised role of NiO in NiOx–STO and in many other nickel-containing water splitting systems, including NiOx–La : KTaO3, and many layered perovskites.

 Please wait while we load your content...
Please wait while we load your content...