Catalytic interconversion between hydrogen and formic acid at ambient temperature and pressure†
Abstract
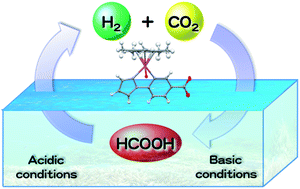
Interconversion between hydrogen and formic acid in water at ambient temperature and pressure has been made possible by using a [C,N] cyclometalated organoiridium complex, [IrIII(Cp*)(4-(1H-pyrazol-1-yl-κN2)benzoic acid-κC3)(H2O)]2SO4 [1]2·SO4, as an efficient catalyst for both directions depending on pH. Hydrogenation of carbon dioxide by hydrogen occurs in the presence of a catalytic amount of 1 under an atmospheric pressure of H2 and CO2 in weakly basic water (pH 7.5) at room temperature, whereas formic acid efficiently decomposes to afford H2 and CO2 in the presence of 1 in acidic water (pH 2.8).
- This article is part of the themed collection: Carbon Dioxide

 Please wait while we load your content...
Please wait while we load your content...