Ligand effect on the catalytic activity of ruthenium nanoparticles in ionic liquids†
Abstract
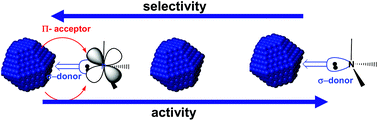
Suspensions of small sized (1–2.5 nm) ruthenium nanoparticles (RuNPs) have been obtained by decomposition, under H2, of (η4-1,5-cyclooctadiene)(η6-1,3,5-cyclooctatriene)ruthenium(0), [Ru(COD)(COT)], in the ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide, [C1C4Im][NTf2], and in the presence of different compounds acting as ligands: C8H17NH2, PPhH2, PPh2H and H2O. Previous and new liquid NMR experiments showed that the ligands are coordinated or in the proximity to the surface of the RuNPs. Herein is reported how the ligand affects the catalytic performance (activity and selectivity) compared to a ligand-free system of RuNPs, when RuNPs in [C1C4Im][NTf2] are used as catalysts for the hydrogenation of various unsaturated compounds (1,3-cyclohexadiene, limonene and styrene). It has been observed that σ-donor ligands increase the activity of the nanoparticles, contrarily to π-acceptor ones.
- This article is part of the themed collection: In Celebration of David Cole-Hamilton's Career in Chemistry

 Please wait while we load your content...
Please wait while we load your content...