The trifluorido complex mer-[CrF3(py)3] (py = pyridine) reacts with 1 equiv. of [Ln(hfac)3(H2O)2] and depending on the solvent forms the tetranuclear clusters [Cr2Ln2(μ-F)4(μ-OH)2(py)4(hfac)6], 1Ln, and [Cr2Ln2(μ-F)4F2(py)6(hfac)6], 2Ln, in acetonitrile and 1,2-dichloroethane, respectively (Ln = Y, Gd, Tb, Dy, Ho, and Er; hfacH = 1,1,1,5,5,5-hexafluoroacetylacetone). Reaction with [Dy(hfac)3(H2O)2] in dichloromethane produces the dinuclear cluster [CrDy(μ-F)F(OH2)(py)3(hfac)4], 3Dy. All the clusters feature fluoride bridges between the chromium(III) and lanthanide(III) centres. Fits of susceptibility data for 1Gd and 2Gd reveal the fluoride-mediated chromium(III)–lanthanide(III) exchange interactions to be 0.43(5) cm−1 and 0.57(7) cm−1, respectively (in the 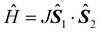 convention). Heat capacity measurements on 2Gd reveal a moderate magneto-caloric effect (MCE) reaching −ΔSm(T) = 11.4 J kg−1 K−1 for ΔB0 = 9 T → 0 T at T = 4.1 K. Out-of-phase alternating-current susceptibility (χ′′) signals are observed for 1Dy, 2Dy and 2Tb, demonstrating slow relaxation of the magnetization.
convention). Heat capacity measurements on 2Gd reveal a moderate magneto-caloric effect (MCE) reaching −ΔSm(T) = 11.4 J kg−1 K−1 for ΔB0 = 9 T → 0 T at T = 4.1 K. Out-of-phase alternating-current susceptibility (χ′′) signals are observed for 1Dy, 2Dy and 2Tb, demonstrating slow relaxation of the magnetization.
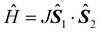 convention). Heat capacity measurements on 2Gd reveal a moderate magneto-caloric effect (MCE) reaching −ΔSm(T) = 11.4 J kg−1 K−1 for ΔB0 = 9 T → 0 T at T = 4.1 K. Out-of-phase alternating-current susceptibility (χ′′) signals are observed for 1Dy, 2Dy and 2Tb, demonstrating slow relaxation of the magnetization.
convention). Heat capacity measurements on 2Gd reveal a moderate magneto-caloric effect (MCE) reaching −ΔSm(T) = 11.4 J kg−1 K−1 for ΔB0 = 9 T → 0 T at T = 4.1 K. Out-of-phase alternating-current susceptibility (χ′′) signals are observed for 1Dy, 2Dy and 2Tb, demonstrating slow relaxation of the magnetization.![Graphical abstract: Fluoride-bridged {Ln2Cr2} polynuclear complexes from semi-labile mer-[CrF3(py)3] and [Ln(hfac)3(H2O)2]](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C2DT31302B&imageInfo.ImageIdentifier.Year=2012)

 Please wait while we load your content...
Please wait while we load your content...