Catalyzed hydrogenation of condensed three-ring arenes and their N-heteroaromatic analogues by a bis(dihydrogen) ruthenium complex†‡
Abstract
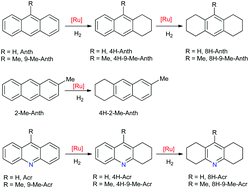
A series of anthracene and acridine derivatives were hydrogenated under mild reaction conditions (80 °C, 3 bar of H2) using the bis(dihydrogen) complex [RuH2(η2-H2)2{P(C6H11)3}2] (1) as a catalyst precursor. The influence of a methyl substituent on the substrate was studied. In all our systems, hydrogenation was only observed at the external rings leading to the corresponding 4H- or 8H-derivatives of anthracene and acridine. Three complexes resulting from the η4(C,C)-coordination of the substrate to the unsaturated fragment [RuH2{P(C6H11)3}2] were characterized. In the case of 9-methyl acridine, the corresponding complex [RuH2(η4-C14H11N){P(C6H11)3}2] (4) turned out to be an active catalyst precursor leading to 1,2,3,4,5,6,7,8-octahydro-9-methylacridine as the sole product after 24 h. Regeneration of 1 from 4 supports the role of complex 4 in the catalytic cycle. Three hydrogenated products, 1,2,3,4-tetrahydroanthracene (4H-Anth), 1,2,3,4-tetrahydro-9-methylanthracene (4H-9-Me-Anth) and 1,2,3,4-tetrahydroacridine (4H-Acr), were characterized by X-ray diffraction.
- This article is part of the themed collection: In Celebration of David Cole-Hamilton's Career in Chemistry

 Please wait while we load your content...
Please wait while we load your content...