Hydroformylation of 1-octene using low-generation Rh(i) metallodendritic catalysts based on a tris-2-(2-pyridyliminoethyl)amine scaffold†
Abstract
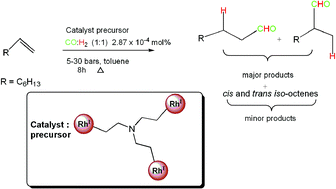
The synthesis and characterization of low-generation pyridylimine Rh(I) metallodendrimers is described. These metallodendrimers were obtained via a Schiff base condensation of tris-2-(aminoethyl)amine with 2-pyridinecarboxaldehyde to afford the tris-2-(2-pyridylimine ethyl) amine ligand (1). Subsequent complexation reactions with [RhCl(CO)2]2 and [RhCl(COD)]2 yielded the corresponding metal-containing dendrimers containing –RhCl(CO) and –Rh(COD) moieties on the periphery. These new rhodium metallodendrimers (2 and 3) and their precursor ligand (1) are thermally stable and have been characterized using 1H NMR, 13C NMR, 31P NMR, FT-IR spectroscopy, elemental analysis as well as mass spectrometry. The Rh(I) metallodendrimers are highly active and chemo- and regioselective in the hydroformylation of 1-octene. Aldehydes were favoured at moderate to high temperatures (95 °C and 75 °C) and pressure (30 bars), while more iso-octenes were formed at low temperature (55 °C) and pressures (5 and 10 bars). The mononuclear analogues (5 and 6) also produced more aldehydes (albeit showing catalyst decomposition at 95 °C and 75 °C, 30 bars) and these aldehydes were mostly branched.

 Please wait while we load your content...
Please wait while we load your content...