Rearrangements of phosphinoimines to phosphine–imines in ruthenium chelate complexes†
Abstract
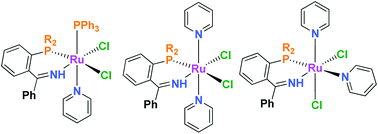
Thermal reaction of 1 : 1 mixtures of the RuCl2(PPh3)3 and phosphinoimine R2PN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 (R = Ph, iPr, Me) at 140 °C results in isolation of the dimeric species [RuCl(μ-Cl)(PPh3)(C6H4(PPh2)C(Ph)NH)]2 (R = Ph 1, iPr 2, Me 3) containing phosphine–
CPh2 (R = Ph, iPr, Me) at 140 °C results in isolation of the dimeric species [RuCl(μ-Cl)(PPh3)(C6H4(PPh2)C(Ph)NH)]2 (R = Ph 1, iPr 2, Me 3) containing phosphine–![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2)(SIMes)(CHPh) 9 and RuCl2(PPh3)2(HN
CPh2)(SIMes)(CHPh) 9 and RuCl2(PPh3)2(HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Ph)C6H4) 10 provide support for a proposed mechanism involving a intermediate containing a Ru-bound metallated aryl-imine fragment.
C(Ph)C6H4) 10 provide support for a proposed mechanism involving a intermediate containing a Ru-bound metallated aryl-imine fragment.

 Please wait while we load your content...
Please wait while we load your content...