Substituent effects on Ni–S bond dissociation energies and kinetic stability of nickel arylthiolate complexes supported by a bis(phosphinite)-based pincer ligand†
Abstract
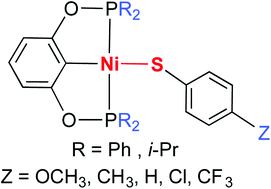
Pincer complexes of the type [2,6-(R2PO)2C6H3]NiSC6H4Z (R = Ph and i-Pr; Z = p-OCH3, p-CH3, H, p-Cl, and p-CF3) have been synthesized from [2,6-(R2PO)2C6H3]NiCl and sodium arylthiolate. X-ray structure determinations of these thiolate complexes have shown a somewhat constant Ni–S bond length (approx. 2.20 Å) but an almost unpredictable orientation of the thiolate
- This article is part of the themed collection: New Talent: Americas

 Please wait while we load your content...
Please wait while we load your content...