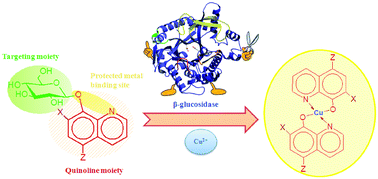
8-Hydroxyquinolines are systems of great interest in the field of inorganic and bioinorganic chemistry. They are metal-binding compounds and are known to exhibit a variety of biological activities, such as antibacterial and anticancer activities. Among these systems, clioquinol has been the focus of a renewed interest in recent years. In this scenario, we synthesized and characterized the new clioquinol glucoconjugate, 5-chloro-7-iodo-8-quinolinyl-β-D-glucopyranoside in order to compare this system to that of clioquinol. We also synthesized, 8-quinolinyl-β-D-glucopyranoside, an 8-hydroxyquinoline glucoconjugate. The reason for the development of glucoconjugates is the glucose avidity, and the over-expression of glucose transporters in cancer cells. Here we demonstrate that glycoconjugates are cleaved in vitro by β-glucosidase and these systems exhibit antiproliferative activity against different tumor cell lines in the presence of copper(II) ions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...