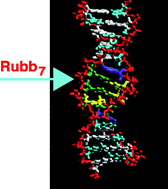
The binding of ΔΔ/ΛΛ-[{Ru(phen)2}2(μ-bbn)]4+ {where phen = 1,10-phenanthroline, bbn = 1,n-bis[4(4′-methyl-2,2′-bipyridyl)]-alkane (ΔΔ/ΛΛ-Rubbn)} to the non-self complementary oligonucleotide 5′-d(CGCGATAAGCCGC·5′-GCGGCATTACGCG) (3-DB) has been examined using a 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) displacement assay. The 3-DB oligonucleotide contains two single adenine bulge nucleotides that are separated by three base pairs. 1H NMR spectroscopy data demonstrated that the adenine bases are intra-helical and that the segment containing the two bulge nucleotides and the three A·T base pairs between the bulges forms a destabilised segment within the stable duplex oligonucleotide. The DAPI displacement assay demonstrated that ΔΔ-Rubb7-bound 3-DB with higher affinity than the other members of the ΔΔ/ΛΛ-Rubbn series. Molecular models suggested that the seven-carbon chain length in ΔΔ-Rubb7 was ideal to span the distance between the two bulge sites. The binding of ΔΔ-Rubb7 to 3-DB was also studied by 1H NMR spectroscopy and molecular modelling. The selective changes in chemical shifts for the resonances from 3-DB upon addition of ΔΔ-Rubb7 suggested that the metal complex specifically bound at the destabilised segment between A5 and A19. Observation in NOESY spectra of NOE cross peaks between 3-DB and ΔΔ-Rubb7 confirmed that one of the ruthenium centres bound at the A5 bulge site, with the other metal centre positioned at the A19 bulge. In addition, ΔΔ-Rubb7 was found to bind chromosomal DNA extracted from a suspension of Staphylococcus aureus that had been incubated with the ruthenium(II) complex. As inert dinuclear ruthenium(II) complexes are capable of being transported into a bacterial cell and bind chromosomal DNA, it is possible that they could be developed into anti-microbial agents that specifically target destabilised segments of DNA that are recognised by essential DNA-binding proteins.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...